Acid Control in Winemaking
Types of acid that occur
Fruits contain a variety of weak organic acids of which only three occur in quantity: tartaric, malic and citric. Grapes contain mainly malic and tartaric acids. Minor acids such as succinic, which contribute to aroma and flavour are also present. The presence of these acids in the correct amounts is a vital factor in determining if the must has the potential to produce good wine. ["must" is word used for the juice or crushed grapes before fermentation]
Malic acid is the preponderant acid of unripe grapes. As grapes ripen the amount of malic acid diminishes. Tartaric acid becomes the principal acid in ripe grapes though plenty of malic may remain. Grape variety and the conditions in which it is grown may produce large differences in the amounts of these acids.
Measuring Acid Concentration
When we measure the TA of a must or wine, we express it as though it were all tartaric. The units we shall use are grams per litre or "g/L". [If you prefer percent, divide by 10].
It is essential to measure the acid content of every must, and if necessary, correct it in order to maximize the potential to yield a good wine. Even if the shipper of the grapes, juice or wine kit includes a measurement of the acid content, it may not necessarily be correct. Do your own testing.
The acid testing kits sold by winemaking stores are easy to use, and do a fairly accurate job. They provide a syringe, a vial of "indicator solution" and a bottle of sodium hydroxide solution, usually 0.2 N. in strength.
You provide a glass container to do the test in. A laboratory beaker is okay. An erlenmeyer flask is even better since it is built for swirling.
Testing White musts or wines
Accurately measure a 15.0 millilitre or "mL" sample into the container and add 4 or 5 drops of indicator solution. Fill the syringe to the 10 mL mark with the 0.2 N. sodium hydroxide solution.
Add sodium hydroxide solution drop-by-drop to the sample. With white musts or wines, you will see that each drop creates a fleeting pink zone which vanishes when the sample is swirled. Keep adding the sodium hydroxide until the pink flush just becomes persistent throughout the whole sample, but does not become bright red. This is your "endpoint".
The number of mL's of sodium hydroxide solution you used is the same as the number of g/L TA. [If you were sold 0.1 N sodium hydroxide solution, instead of 0.2 N, the number of g/L TA is half the number of mL of sodium hydroxide solution used.]
Testing Reds
The test for red must or wine is the same, except that due to the colour, the endpoint is more difficult to see, sometimes nearly impossible.
When titrating reds, work with a brightly lit background. As you add sodium hydroxide solution, the red of the grapes will gradually disappear and turn to a greyish shade. When the sample is a definite greyish - greenish shade, you are close to the endpoint. The endpoint is reached as a reddish tinge reappears due to the colour change of the indicator solution.
Unless you are confident about your ability to correctly interpret these colour changes, you should use one of the alternative methods of testing reds discussed later.
Shelf-life of Sodium Hydroxide Solution
Sodium hydroxide solution keeps for several months, but will eventually lose strength as air (specifically carbon dioxide) is admitted to it. Date each bottle as you buy it and keep it well closed.
You can test your sodium hydroxide by using a solution of 5.0 grams of tartaric acid made up to 1.0 litres with water. Using a 15 mL sample of this, check its TA as you would for wine. It should, of course, read 5.0 g/L. If it reads much over this - for example, a reading of 6 would mean your measurements will be 20% too high - then get fresh sodium hydroxide solution.
Making up your own Acid Testing Solutions
The 0.2 N sodium hydroxide solution is made by accurately weighing out 8.0 grams of solid sodium hydroxide and adding sufficient water to it to bring the total volume to 1.0 litres.
Solid sodium hydroxide is difficult to handle. It is extremely corrosive. It also absorbs water from the air very rapidly, thus the weighing must be done quickly. Solid sodium hydroxide must be stored in a very well sealed air tight container, otherwise the individual pellets will turn into one unmanageable lump.
The indicator solution is made by dissolving 1 gram or so of solid phenolphthalein in roughly 100 mL of dilute alcohol. It need not be particularly accurate.
The "White Tile" Method for measuring TA in Red Wines - Testing to the nearest gram per litre.
Here is another technique that makes it easier to see the colour changes when titrating red wines. It sounds excessively complicated when you first read it, but after you’ve gone through it step by step you’ll see how nicely it works.
Get an approximately six inch by six inch matte white glazed ceramic tile and mark on it an arc of 9 - three quarter inch diameter circles and a central control circle using a permanent felt pen. Label the circles in series to cover the TA range you wish to examine. [For example: 4, 5, 6, 7, all the way to 12 g/L.]
Place a drop of phenolphthalein indicator solution at the centre of each circle except the control circle.
Measure 15.0 mL of red must or wine into a clean beaker or flask. Don’t add indicator solution to the wine.
Using a stirring rod made of glass, plastic or some other non-absorbent material, place one drop of the wine onto the control circle. This serves as a comparison with whatever colour changes occur.
Fill a clean dry syringe with 10.0 mL of 0.2N sodium hydroxide solution. Add sodium hydroxide to the wine, mixing with the stirring rod, until you have reached the number of mL of sodium hydroxide corresponding to the lowest number of your marked circles [in our example, 4mL].
Stir the wine sample again, just to make sure it's well mixed, then use the rod to place a drop of it onto the indicator solution in the circle marked with the lowest number [again, in our example, the first circle which is labelled 4].
Continue by carefully adding 1.0 mL of sodium hydroxide solution at a time to the sample.
At each 1.0 mL addition thoroughly stir the wine with your applicator rod then place a drop of wine onto the circle with the next highest number.
As you approach the endpoint your sample will lose its red colour and will be turning a greyish green shade.
When you arrive at, or pass the end point there will be a distinct change in the colour of the drop of wine as soon as it is placed on its circle. The greyish green shade will change to red in the drop of indicator.
When you reach this point you will know that the precise end point lies between the value of this circle and the one previous.
Refining the test to the nearest tenth of a gram per litre
Now repeat the test with a fresh 15.0 mL wine sample and a full 10.0 mL syringe of 0.2 N. sodium hydroxide solution.
This time number the circles 0.1, 0.2, 0.3 up to 0.9 g/L and place a drop of indicator on each as before. Again, do not add indicator to the main sample.
Place a drop of the wine on the control circle before adding any sodium hydroxide.
Add enough sodium hydroxide to the wine sample to bring it to 1.0 mL below the number of the circle where the indicator solution turned red in the first part of the test. [If for example circle 6 had gone greyish and circle 7 had showed the indicator solution change to red, you would add 6.0 mL .]
Now add a further 0.1 mL sodium hydroxide, stir with the applicator rod and place a drop in the circle marked 0.1. Add another 0.1 mL and test it on the circle marked 0.2.
Continue in this manner until a drop of sample turns red when it is placed on the indicator solution on the tile. Read the value of this circle and add it to the value where you started this second test. [If in our example if the circle marked 0.4 turned red, the TA would be 6.4 g/L]
Rosé musts
For rosé wines use whichever technique you find best.
Appropriate acid levels
In a must for making a white wine the TA should be roughly in the 7 to 9.5 g/L range, and can go higher under some circumstances. For instance, musts for dessert or champagne type wines can sometimes be well over 10 g/L.
The TA of red wine musts should generally be in the 6 to 8 g/L range.
Sometimes you will find a must with too low a TA.
You can raise TA by adding tartaric acid. Calculate the quantity on the basis that 1 gram will raise TA of a litre of white must by 1.0 g/L. Because of the presence of large quantities of solids in red musts less acid will be needed than for whites. You should add a fraction of what you think you’ll need, dissolved in some juice and well mixed; re-test and adjust accordingly.
Musts from unripe grapes are often too high in TA.
If TA is at 10 g/L or lower it is probably best to ferment it as is, possibly involving a malolactic fermentation, and to deal with any excess acidity later.
If the TA is much above 10 g/L you may find it difficult to make a decent table wine. Dilution with water, blending with low acid concentrate or juice, or treatment with "Acidex" or potassium carbonate are some possibilities.
Acid, TA and pH
Acidity is due to the presence of hydrogen particles. In a must or wine these occur in both free and bound, states.
pH is a measurement of how many free positively charged hydrogens are around.
When measuring TA, you add sodium hydroxide to the must. What happens is, you deal with the free hydrogens, those responsible for the pH. But even as you do and as you continue to add more sodium hydroxide, you actually start to unhook bound hydrogens and make them free.
Only when you have added sufficient sodium hydroxide to unhook all accessible hydrogens is your measurement of TA complete.
Since the proportions of free and bound hydrogens varies greatly according to grape varietal, ripeness, growing conditions and so on, so does the relationship between pH and TA.
Thus, the two numbers, TA and pH, in a must or wine have no direct or predictable relationship. The best one can do is to say that higher TA is generally associated with lower pH and vice versa.
The importance of pH
pH is at least as important as TA in a must. It has a powerful effect upon the efficiency of sulphur dioxide and upon the ability of malolactic bacteria and wild yeasts to function in the must or young wine. For various chemistry type reasons, pH 3.55 is considered the magic dividing line between relative safety and more vulnerability to problems of oxidation and/or undesirable bacterial infection.
Acceptable pH values
Partially ripened grapes often combine a high TA with a very low pH. Over ripe grapes often have a low TA with too high a pH. Occasionally one encounters grapes with normal or high TA and a high pH, well above the 3.55 level - such grapes are difficult to work with.
The must for a white wine should ideally start in the pH 3.2 to 3.4 range. Red musts from pH 3.3 to 3.5. These numbers are pretty arbitrary but in general if the pH of your must falls within the ranges indicated, you will have an easier time controlling oxidation, acid balance and bacterial activity.
Measuring pH
In the big wineries pH is measured by elaborate pH meters that can cost thousands of dollars. Home winemakers can obtain a pH "pen" for around $50 to $80 that will be accurate to plus or minus 0.1 pH units. For most purposes, this will be satisfactory. "Self calibrating" pH pens that read to plus or minus 0.01 pH units are more expensive - in the hundreds of dollars - and are very nice if you can afford one.
Litmus or pH papers are virtually useless.
The indispensable partner of a pH pen is one - or more - buffer solutions.
Each is a carefully prepared solution of chemicals having a known and stable pH. Each is used as a standard by which the pH pen can be tested and adjusted.
Chemical supply firms sell these either as solutions or as capsules of powder. In the case of the capsules, you dissolve the contents in a predetermined volume of distilled water to produce the buffer solution.
The buffer chosen should have a pH fairly close to the range expected in wines or musts. A pH 3 or pH 4 buffer is a good choice. A pH 7 buffer is also useful for calibrating the pen when it is used to measure TA. ("Self calibrating" pH pens generally require two buffers: one at pH 4.01 and one at pH 7.00.)
You must "condition" and calibrate a new pH pen by immersing its tip - with its switch "on" - in a buffer for at least 30 minutes prior to use.
If the digital readout shows a value other than that of the known pH of the buffer being used - then the pen must be adjusted by inserting a small screwdriver into the hole beside the pocket clip on the back of the pen. With the pen tip still in the buffer, rotate the screwdriver until the readout is correct for the buffer being used.
It is important to check the calibration of your pH pen at the start of every work session using fresh uncontaminated buffer. Never let the tip of the pen dry out.
Don’t immerse the pen into any liquid past the ridge that the protective cap bears upon. or lay the pen flat - either act may permit liquid to damage the electronics of the pen.
After calibration or checking, shake excess buffer off the pen sensor tip then move it directly to the wine to be tested.
Allow at least 30 seconds for the pH reading to settle.
Never return used buffer to the stock bottle. Dump it out. Don't keep buffer more than two or three months.
Also don’t use tap water as a buffer or to prepare buffers. It will badly mislead you.
After using the pH pen rinse the tip in distilled water, then replace the protective cap. In the cap you should keep a little wad of paper towel or plastic foam moistened with some of the buffer in use or better still a solution saturated with both potassium chloride and silver chloride.
Store the pH pen somewhere where it is held securely upright.
The battery in the pen will take at least a thousand readings provided the pen is not put away with its switch in the "on" position. The fragile electrode in the sensor tip is usually stated to have a useful life of two years or so. By that time the pen will have saved you its price in wines several times over.
Adjusting pH downward
If the pH of your must is too high, 3.6 or more it can be lowered by the addition of straight tartaric acid. (Acid blends create more problems than they solve and should be avoided.)
Get a 1.0 litre test sample of must and stir in 0.5 grams of tartaric acid. Make sure the acid is completely dissolved before re-testing the pH. It should be lower. If necessary, add further 0.5 additions of tartaric acid, testing as you go. When you reach a satisfactory pH in your 1.0 litre sample, calculate the amount of acid to add to your main batch.
The pH of most musts reacts readily to the acid addition, but some can be very stubborn. However, even if you find you are pushing your TA a bit high in order to get the pH down, remember that tartarate can removed after fermentation by cold stabilization or if absolutely necessary by the addition of potassium carbonate.
During fermentation there is sometimes a decrease in TA and an increase in pH. Monitor the pH during fermentation especially with red wines, and add tartaric acid as needed to hold pH in line until fermentation is completed. (Testing TA during fermentation is difficult due to interference from CO2.)
Measuring TA with the help of a pH pen
A pH pen has another very convenient use. As we saw earlier on it is often hard to measure the TA of a red wine or must because of the difficulty of seeing the end point while titrating. Avoid this problem by using the pH pen.
The technique is simple. Although it isn't absolutely necessary, you will get more accuracy if you re-calibrate your pH pen using a pH 7 buffer. Measure out a 15 millilitre sample of the red wine or must to be tested and place it in a beaker.
Fill a syringe with 0.2 N sodium hydroxide. With the pH pen held in the sample, start adding sodium hydroxide while continuously swirling the container.
You will observe that the digital reading of the pen soon begins to climb. You need to take it up until it reads pH 8.2, adding drops very slowly as you approach that point.
The number of millilitres of sodium hydroxide used to reach pH 8.2 gives you the TA in g/L.
Malolactic Fermentation –"MLF".
A "purist" once pointed out to me that before inducing a malolactic fermentation, I should think about the fact that malic acid is part of the grape. Lactic acid is not - rather it is a product of bacterial action and thus a contaminant. I resisted the urge to retort that alcohol is not part of the grape either. So - enough philosophy and on to practicalities.
The two main acids found in grapes are tartaric and malic. Of the two, malic has a more aggressive acid effect on the palate. Lactic acid, the product of malolactic fermentation is less "sour" than either. During malolactic fermentation (MLF), malic acid is converted into lactic acid and carbon dioxide (CO2): 1 gram (g.) of malic acid will be converted into 0.7 g. of lactic plus 0.3 g. of CO2. Thus the effect on the acidity is to produce a less aggressive acid and a smaller quantity of it. MLF will also increase the complexity of a wine. The flavour becomes more mellow, the nose more complex and vinous.
To have an MLF occur is desirable in many red wines and in some whites, such as Chardonnay or Sauvignon Blanc. MLF should probably be avoided in wines whose appeal is their fresh fruity characteristic such as Riesling or Gewürztraminer. If you are aiming for a crisp fruity Sauvignon Blanc or Chardonnay, MLF should be avoided here as well. A compromise, a partial MLF is possible but a bit tricky for the hand-winemaker- we’ll talk about it later, along with how to avoid MLF altogether.
Just as you need a hydrometer to track the progress of your alcohol fermentation, so you need a means of checking on the progress of an MLF. This is paper chromatography and it’s rather more complicated than taking a hydrometer reading. You need either to put together a paper chromatography kit, or to purchase one from your winemaking supplier. I’ll tell you about making or refreshing the solvent at the end of this article.
There are two "chromatograms" illustrated. You should refer to them as you read how to set one up.
(Click the thumbnail for a larger picture.)
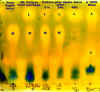 First, draw a line with a pencil (ink will run and ruin things) about 2 cm above the bottom edge of the long side of the special paper included in the kit. Pencil six or seven "X’s" equally spaced along the line and label each with the name of the samples to be tested. Add the date of the tests.
First, draw a line with a pencil (ink will run and ruin things) about 2 cm above the bottom edge of the long side of the special paper included in the kit. Pencil six or seven "X’s" equally spaced along the line and label each with the name of the samples to be tested. Add the date of the tests.
Fold a couple of sheets of ordinary typing paper into roughly 2-cm pleats accordion style. Lay the chromatography paper on one of these and use the other to hold the capillary tubes. Fill a capillary for each of the samples to be tested by dipping it in the liquid until it rises pretty well to the top of the tube. Touch the end of each tube briefly to the "X" mark it belongs to. Make sure the wet patch formed is a maximum 1-centimeter across. Let the patches dry then re-apply the capillaries. Continue this process until the capillaries are empty. In this way you create a concentrated patch for each sample. When the spots are dry following the final application, staple the short sides of the chromatography paper together to form a cylinder. Handle the paper by the edges - acidic fingerprints could interfere.
Pour about a centimeter of the yellow chromatography solvent into the bottom of the jar supplied (about 100 mL). Lower the cylinder of chromatography paper, "X" marks downwards, into the jar, and seal it.
It will take about 8 hours for the solvent to get near the top of the paper. As the chromatography solvent makes its way up, it carries the various acids with it. The lactic acid, marked L on the pictures, moves the furthest. Second furthest, marked M, is the malic acid. Tartaric acid moves the least of all and is marked with a T. When the solvent is close to the top (don’t let it go past), take the cylinder out and put it upright on a piece of paper towel somewhere well ventilated to dry. It will take a day or even more for the colours to completely develop, depending on temperature and air circulation. When it is done, you will see yellow patches representing the acids against a greenish background.
If you are going to get an MLF going in a wine, the first thing to do is to get a culture of ML bacteria. One product available on the market is a blend of cultures of the bacterium leuconostoc oenos. It comes in liquid form in a 114-mL packet, and should be stored under refrigeration. The instructions say the packet is sufficient for 5 gallons of wine. You may have more than that and will need to increase the amount of culture.
Follow the progress of a culture illustrated by the first illustration: The first sample is from a 1-litre package of apple juice. Make sure there are no preservatives such as sodium benzoate. Vitamin C is okay. You can see from the chromatogram that the main acid component of the apple juice is malic. The second sample is directly from the packet of culture. This shows a high proportion of lactic acid at the top, with some malic still left.
 Mix the culture and apple juice in a 2 or 3 liter bottle under as sterile conditions as you can. Sample 3 was taken from such a mix immediately after it was made. Cover the bottleneck with saran wrap and a rubber band, to keep dust and insects out, but also to allow CO2 to escape. Place it in a warm spot where the temperature will be around 25°C. Sample 4 shows that after 24 h. some of the malic acid of the apple juice has turned to lactic, but not much. Sample 5 shows that after 48h. all the malic acid has been converted to lactic and the culture is ready.
Mix the culture and apple juice in a 2 or 3 liter bottle under as sterile conditions as you can. Sample 3 was taken from such a mix immediately after it was made. Cover the bottleneck with saran wrap and a rubber band, to keep dust and insects out, but also to allow CO2 to escape. Place it in a warm spot where the temperature will be around 25°C. Sample 4 shows that after 24 h. some of the malic acid of the apple juice has turned to lactic, but not much. Sample 5 shows that after 48h. all the malic acid has been converted to lactic and the culture is ready.
Sample 6 is of a wine that has completed its MLF. It is shown to indicate where the tartaric acid, which is not present in the ML culture shows up.
It is interesting to note that there is a faint indication of acid below the malic position in samples 1, 3, 4 & 5 that does not appear in sample 2. This could be due to the vitamin C (ascorbic acid) or others appearing as traces in the apple juice. If you had added citric acid (possibly in an acid blend) it would also show up between the tartaric and malic spots.
The ML bacteria do best in warm temperatures, under higher pH conditions and an absence of SO2. Since conditions ideal for the MLF conflict with those for the wine itself, compromises are necessary. The bacteria will still work at pH levels down to 3.0 or so, but more slowly. The same is true of temperatures down to about 13°C.
Free SO2 levels should be at a maximum of 15 ppm. If you are going to sulphite the must before adding yeast, try to keep the SO2 under about 25-ppm if possible. After the alcohol fermentation is well underway, much of the free SO2 will have gone. This is a good time to add the ML culture. This is particularly true of red wines where you are probably going to take the fermentation temperature up quite high.
Usually white wines you are going to do an MLF on will be low pH, and will thus will have needed much less SO2 to begin with.
Depending on conditions then, an MLF could take as short a time as a couple of weeks, or as much as several months. Back in the pre-scientific days, winemakers noticed that wines "came alive" again in the spring following their harvest and initial fermentation. This would undoubtedly have been due to MLF caused by naturally present bacteria, combined with the warming temperatures.
If you are using a barrel and you have just taken out a wine that has completed MLF, the new wine you replace it with will almost certainly do an MLF if it hasn't done so earlier.
Now look at the second illustration, the one with samples from six different wines.
The main feature is that wines #s 1, 3 and 5 have completed MLF, while #s 2, 4 and 6 have not. Of those uncompleted, #2 is furthest along with #6 the least.
There is other information to be gleaned, although a person has to be a bit careful not to over interpret.
The tartaric spot on 4 is weak. This is B. C. red wine. There is a possibility of a very high proportion of the acid being malic. In this sort of situation, you need to keep a close eye on the pH and TA. If the acid was in balance before the ML started, a complete MLF could leave the wine short of acid. By the same token, if a wine were heavy on the tartaric with little malic showing, an MLF wouldn’t have much effect.
If the spotting was carefully controlled, that is to say, each capillary equally filled and each spot the same diameter, then wine #6 has more tartaric than the others, and even though ML has started, it looks like more malic was present at the start, as well. In fact #6 is a 1996 B. C. Pinot Blanc with a relatively high TA.
Wine # 5, a 1996 Spenker Ranch Zinfandel is interesting for the tight definition of the tartaric and lactic patches and the lack of indications of the presence any other (minor) acids.
If you want to avoid having an MLF occur, prompt racking off fermentation solids helps, but most important is maintaining adequate SO2 levels. Generally this means a concentration of molecular SO2 of at least 0.8-ppm. The amount of free SO2 to achieve this depends on pH, for instance a minimum of 30 ppm free SO2 at pH 3.3, 40 ppm at pH 3.4. Since some of the SO2 you add immediately becomes bound you need to check to see if you have enough SO2 left in the free state.
Disaster can strike a sweet wine stabilized with potassium sorbate. If SO2 levels are too low and MLF occurs under these conditions, a revolting geranium like smell and flavour develops and the wine has to be dumped.
Suppose you wish to have a Chardonnay that has some complexity, but that also retains a lot of crisp fruity character. A partial MLF is the answer. You can’t partially ferment your whole batch. What you have to do is a complete MLF on a portion of the wine, no MLF on the rest, and subsequently blend. The problem here is preventing the MLF portion from getting the non-MLF portion started. Commercially, this is achieved by sterile filtering the ML bacteria out. This is difficult for the home winemaker. However, if good filtration is done and, most importantly, the molecular SO2 level is kept at a minimum of 0.8 ppm a blend of MLF and non MLF portions should remain stable.
Some notes on your malolactic chromatography kit.
The paper is Whatmans #1 Chromatography paper. Whatmans # 1 Filter paper will also work well, but it is more fragile. It is also cheaper.
The solvent is made as follows: Thoroughly shake together in a 250 mL separatory funnel, 100 mL distilled water, 100 mL n-butanol, 11 mL glacial (concentrated and very nasty stuff to handle*) formic acid and enough bromcresol green indicator (about 0.15 gram) to give a deep yellow colour. Allow to settle. Drain off and discard the bottom, water-soluble portion and you will be left with about 100 mL solvent. *If you are not trained in working with hazardous chemicals, don't even think of working with glacial formic acid yourself. Get someone competent to do it for you.
The solvent can be used several times, but it eventually wears out and the patches from the different acids begin to run into each other. However, don't throw your tired solvent out. You can rejuvenate it by adding n-butanol to bring the volume back to 100 mL, a tad of bromcresol green if necessary for good colour, 100 mL distilled water, 11 mL glacial formic acid, and going through the mixing and separating process all over again. I prefer to make fresh solvent each time I do a chromatogram.
You can make up the solvent without using a separatory funnel. Use a cylindrical container for mixing and allowing the water soluble part to settle to the bottom. Use a syringe to carefully draw off the solvent layer from the top, making sure you don’t pick up any of the bottom layer. It is even possible to get away without having capillary tubes for spotting. Use toothpicks.
Charles Plant 2001
